Binimetinib
CAS No. 606143-89-9
Binimetinib ( MEK162, ARRY-162, ARRY-438162 )
Catalog No. M15269 CAS No. 606143-89-9
Binimetinib (MEK162, ARRY-162, ARRY-438162) is a potent inhibitor of MEK1/2 with IC50 of 12 nM in a cell-free assay. Phase 3.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 32 | In Stock |


|
| 10MG | 51 | In Stock |


|
| 25MG | 61 | In Stock |


|
| 50MG | 72 | In Stock |


|
| 100MG | 87 | In Stock |


|
| 200MG | 140 | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameBinimetinib
-
NoteResearch use only, not for human use.
-
Brief DescriptionBinimetinib (MEK162, ARRY-162, ARRY-438162) is a potent inhibitor of MEK1/2 with IC50 of 12 nM in a cell-free assay. Phase 3.
-
DescriptionBinimetinib (MEK162, ARRY-162, ARRY-438162) is a potent inhibitor of MEK1/2 with IC50 of 12 nM in a cell-free assay. Phase 3.(In Vitro):In MCF7 cells, RSK3 or RSK4 expression decreases response to treatment with any of the PI3K inhibitors alone. However, the combination of PI3K inhibition with Binimetinib (MEK162) or BI-D1870 completely reverses the resistance of RSK-expressing cells. Binimetinib (MEK162) blocks basal ERK phosphorylation in all HRAS mutant cell lines. The combination of RAD001 and AZD6244/MEK162 causes a stronger inhibition of S6 kinase than single use of RAD001 on Western blot. The combination of RAD001 and AZD6244/MEK162 also translated in a stronger blockade of cell growth in HRAS mutant cells than single use. Binimetinib (MEK162) shows stronger synergism with RAD001 than AZD6244. (In Vivo):Treatment with Binimetinib (ARRY-438162) reduces disease severity in a dose-related manner in both animal models. ARRY-438162 in the CIA model inhibits increases in ankle diameter by 27% and 50% at 1 and 3 mg/kg, while Ibuprofen has 46% inhibition. When combined with Ibuprofen, these same two doses result in 74% and 72% inhibition, respectively. Microscopic examination of the ankle joints show Binimetinib (ARRY-438162) significantly inhibits lesions (inflammation, cartilage damage, pannus formation and bone resorption) by 32% and 60% at 1 and 3 mg/kg, while treatment with Ibuprofen alone results in 17% inhibition, which is not significantly different from the controls. When these two doses of Binimetinib (ARRY-438162) are combined with ibuprofen, the result is 54% and 77% inhibition of joint destruction. In AIA, 3 and 10 mg/kg of Binimetinib (ARRY-438162) inhibit AIA ankle diameter 11% and 34%, while MTX has 33% inhibition. When combined with MTX, 3 and 10 mg/kg of Binimetinib (ARRY-438162) result in 55% and 71% inhibition. Microscopic examination of ankle joints for inflammation and bone resorption also shows improved efficacy versus either compound alone. When Binimetinib (MEK162) is combined with BEZ235, a significant reduction of tumor growth is observed (P=0.01). This increase in antitumor activity is accompanied by a decrease in phospho-ERK and phospho-S6 staining. No significant changes are observed in phospho-4EBP1 staining, a direct target of mTOR activity.
-
In VitroIn MCF7 cells, RSK3 or RSK4 expression decreases response to treatment with any of the PI3K inhibitors alone. However, the combination of PI3K inhibition with Binimetinib (MEK162) or BI-D1870 completely reverses the resistance of RSK-expressing cells. Binimetinib (MEK162) blocks basal ERK phosphorylation in all HRAS mutant cell lines. The combination of RAD001 and AZD6244/MEK162 causes a stronger inhibition of S6 kinase than single use of RAD001 on Western blot. The combination of RAD001 and AZD6244/MEK162 also translated in a stronger blockade of cell growth in HRAS mutant cells than single use. Binimetinib (MEK162) shows stronger synergism with RAD001 than AZD6244.
-
In VivoTreatment with Binimetinib (ARRY-438162) reduces disease severity in a dose-related manner in both animal models. ARRY-438162 in the CIA model inhibits increases in ankle diameter by 27% and 50% at 1 and 3 mg/kg, while Ibuprofen has 46% inhibition. When combined with Ibuprofen, these same two doses result in 74% and 72% inhibition, respectively. Microscopic examination of the ankle joints show Binimetinib (ARRY-438162) significantly inhibits lesions (inflammation, cartilage damage, pannus formation and bone resorption) by 32% and 60% at 1 and 3 mg/kg, while treatment with Ibuprofen alone results in 17% inhibition, which is not significantly different from the controls. When these two doses of Binimetinib (ARRY-438162) are combined with ibuprofen, the result is 54% and 77% inhibition of joint destruction. In AIA, 3 and 10 mg/kg of Binimetinib (ARRY-438162) inhibit AIA ankle diameter 11% and 34%, while MTX has 33% inhibition. When combined with MTX, 3 and 10 mg/kg of Binimetinib (ARRY-438162) result in 55% and 71% inhibition. Microscopic examination of ankle joints for inflammation and bone resorption also shows improved efficacy versus either compound alone. When Binimetinib (MEK162) is combined with BEZ235, a significant reduction of tumor growth is observed (P=0.01). This increase in antitumor activity is accompanied by a decrease in phospho-ERK and phospho-S6 staining. No significant changes are observed in phospho-4EBP1 staining, a direct target of mTOR activity.
-
SynonymsMEK162, ARRY-162, ARRY-438162
-
PathwayMAPK/ERK Signaling
-
TargetMEK
-
RecptorMEK
-
Research AreaCancer
-
Indication——
Chemical Information
-
CAS Number606143-89-9
-
Formula Weight441.23
-
Molecular FormulaC17H15BrF2N4O3
-
Purity>98% (HPLC)
-
SolubilityDMSO: 88 mg/mL (199.44 mM)
-
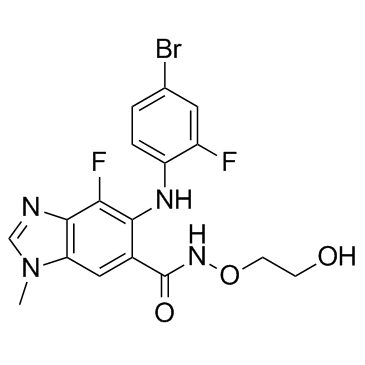
SMILESO=C(C1=C(NC2=CC=C(Br)C=C2F)C(F)=C3N=CN(C)C3=C1)NOCCO
-
Chemical Name5-((4-bromo-2-fluorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzo[d]imidazole-6-carboxamide.
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1. SS Bhagwat, et al. Annu Rep Med Chem, 2007, 42, 265–278.
molnova catalog



related products
-
AZD8330
AZD8330 (ARRY-424704;ARRY-704) is a potent, selective, non-ATP competitive MEK1/2 inhibitor with IC50 of 7 nM.
-
Refametinib R enanti...
The R enantiomer of Refametinib, a potent, non–ATP-competitive, highly selective, allosteric inhibitor.
-
Trametinib DMSO solv...
A potent and highly specific MEK1/2 inhibitor with IC50 of 0.92 nM/1.8 nM.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


